REACH
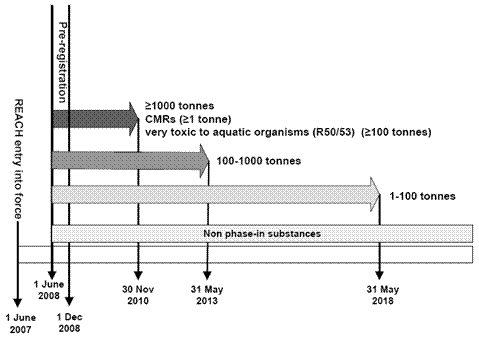
REACH (Registration, Evaluation, Authorisation and restriction of CHemicals) is a regulation of European Parliament and the Council (EC) no. 1907/2006 dated 18 December 2006 which entered into force on 1 June 2007. It concerns chemicals manufactured or imported in EU in quantities exceeding 1 tonne per year which need to be gradually registered by 31 May 2018.
Who, where and how to register?
The responsibility to register chemicals under REACH lies with their producers and importers. In particular, the regulation covers:
- producers, importers and downstream users of substances as such
- producers, importers and downstream users of substances contained in preparations
- producers, importers and downstream users of substances contained in articles
The substances as such or contained in preparations or articles are not allowed to be produced in, or imported to, the EU countries after December 1, 2010 unless they had been registered with the European Chemicals Agency (ECHA) residing in Helsinki. Every producer or importer is due to submit its registration dossier (according to Regulation (EC) No 1907/2006, Annex II) to the Agency if the amount of the substance is more than (or equal to) 1 metric ton per year, or if the substance is listed in Regulation (EC) No 1907/2006, Annex IV or V.
Information submitted with the registration depends on the tonnage of manufactured or imported substance (see Regulation (EC) No 1907/2006, Annexes VII, VIII, IX and X). A dossier (technical documentation) is required to be submitted as part of the registration process. It includes information on physical-chemical properties as well as toxicological and ecotoxicological information of the manufactured or imported substance and also a Chemical Safety Report (CSR) documenting chemical safety risk assessments in accordance with Regulation (EC) No 1907/2006, Annex I. The dossier is to be submitted separately for each substance contained in the preparation or article with registration tonnage over 10 tonnes. Evaluation of the dossiers is carried out by ECHA and substances are not allowed to be used until the evaluation process has been completed and registration number has been assigned to the substance. If the substance does not pass the evaluation process, production or import are not allowed.
Using the pre-registration data, so called SIEF (Substance Information Exchange Forums) began to be formed after January 1, 2009. Companies associated with the SIEF share and assess data and prepare joint parts of registrations (joint submission of data) which are submitted by the Lead Registrant. In the end, each company will submit its own registration dossier containing its specific information that needs to be in line with joint parts of the registration. At the same time, a registration fee to ECHA is paid. Companies within SIEF share the testing costs for studies proportionally.
When to register?
Producers and importers should have used the opportunity to pre-register their substances between June 1 and November 30, 2008. Provided they did so, they are obliged to register the substances within the deadlines set by Regulation (EC) No 1907/2006, Article 23. The deadlines depend on the tonnage of manufactured or imported substance and the degree of its hazardousness. Unless the producer or importer pre-registered the respective substance, he is not allowed to produce it or place it in the EU market as of December 1, 2008. Furthermore, he is obligated to submit the registration dossier in compliance with Regulation (EC) No 1907/2006, Annex II and not to start with the production or import before the registration is carried out.
Enaspol and REACH
As one of the European producers of chemicals, Enaspol meets all obligations associated with the REACH legislation. That is, by 1 December 2008 we pre-registered all our substances that are not exempt from the registration and are manufactured in quantities over 1 tonne a year. Subsequently, within the respective deadlines (2010 and 2013), we registered all our substances produced in quantities over 1000 t/a and 100 t/a, respectively, as members of joint submission. At present we work on registration of the remaining substances.
Address
Enaspol a.s.
Velvěty 79
Velvěty 79
Rtyně nad Bílinou
415 01
415 01
Call us
+420 417 813 111
+49 1520 3455096
Fax us
+420 417 532 560
 Čeština
Čeština
 Print page
Print page